Answer:
Option D
Explanation:
According to question , we can draw the following diagram
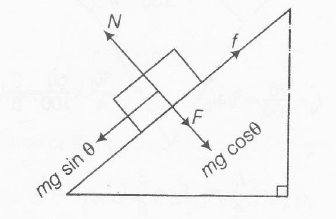
mg \sin \theta= \mu (F+ mg \cos \theta)
F= \frac{mg \sin \theta}{\mu}-mg \cos \theta
F=mg\left[\frac{\sin \theta}{\mu}-\cos \theta\right]
Here,
m=1kg ,g=10 m/s2, \theta=30^{0}, \mu=0.2
F=1\times 10\left[\frac{\sin 30}{0.2}-\cos 30^{0}\right]
= 10\left[\frac{1}{2\times 0.2}-\frac{\sqrt{3}}{2}\right]
= 10\left[\frac{5}{2}-\frac{\sqrt{3}}{2}\right]=5[5-\sqrt{3}]
=5[5-1.732]=16.34 N